Calculating the Karat Content of Gold and Interpreting Marks
This article will hep you understand how to calculate karat content of gold, what to watch out for, and what the various marks on gold jewelry mean.
6 Minute Read
So how do you figure the karat content of the item? You need an accurate scale to determine how much a piece of gold or gold jewelry item weights. But it's the amount of gold that presents a problem… 10K gold or 14K gold or 18K gold or 24K gold - or gold plated or gold filled.
Because real gold is involved, let's first examine what is meant by gold filled and gold plated - and then proceed to the relatively easy technique for testing scrap gold karatage.
"Gold filled" is a term which indicates a manufacturing process whereby two thin gold sheets with a supporting piece of core metal forms a sort of metal sandwich construction which is then laminated with a brazing alloy into one inseparable sheet.
After this so-called fused ingot is made it is placed in a rolling mill and rolled out to the desired thickness. This filling process must be accomplished mechanically in order for the process to be identified as gold filled.
Stamping-under the new amended federal act - indicates the gold content of the sandwich by weight and the karatage. Thus, a GF article stamped "1/20-14K" means that at least 1/20 of the total metal content is comprised of gold and that the gold sheet used was 14K.
Gold plating is an electrolytic process is which direct current is used to deposit gold on a host metal. As with gold filled items, the stamping usually indicates the quality by weight in proportion to the weight of the article and the karat of the plate.
Many gold plated articles are not marked with the percentage of the weight of the gold plate. They are marked only with the karat, for example: "14K G.P." or "10K Gold Plate."
Various markings are found in the Gold Filled and Gold Plated area. Many items contain marks such as R.G.P. for "rolled gold plate" or HGP for "Heavy Gold Plate."
Old gold watched with gold plating rarely are marked with the weight or the karat value. The watches were so heavy that they could not be covered economically with gold so as to constitute 1/10 or 1/20 of the weight by gold.
These old watches are valuable, though. You can often find markings that indicate the probable number of years of expected wear… 10 year, 20 year and 25 year cases.
Lately, the jewelry industry has been utilizing gold and silver epoxies for low cost jewelry items. The technology here is so advanced that these epoxies need a careful look - and the y often wear better than gold plating.
How do you know, then, if that old ring with no markings is gold? And if it is gold, how do you know if it's the minimum of 10K in order to be called gold - or if it's 14K, 18K, or 24K? And is there a way to determine if that expensive looking gold piece is gold filled, gold plated, or epoxied. Yes.
There is a refined laboratory technique using a series of chemicals.
There is also a much simpler, less expensive way that was developed and announced by Harry Landis, Inc., a New York refiner.
Let's consider the Landis procedure.
The necessary equipment involves nitric acid. Before going any further, let there be a firm warning at the point: nitric acid is a must to test gold, but nitric acid when used carelessly is very dangerous. If handled with care, nitric acid will cause no problems.
Jewelers and precious metal specialists have used nitric acid for centuries with very few incidents, accidents or casualties. But this powerful acid can result in serious pain and injury, and will damage almost anything with which it comes in contact.
It is poisonous and deadly if ingested, even in trivial amounts. You should use it only in a well ventilated area, and wear rubber gloves. Water - for a quick emergency rinse - should be kept readily available. The author and publisher take no responsibility whatsoever for any consequences if or when you use nitric acid, and you take full responsibility for damages to yourself or to others.
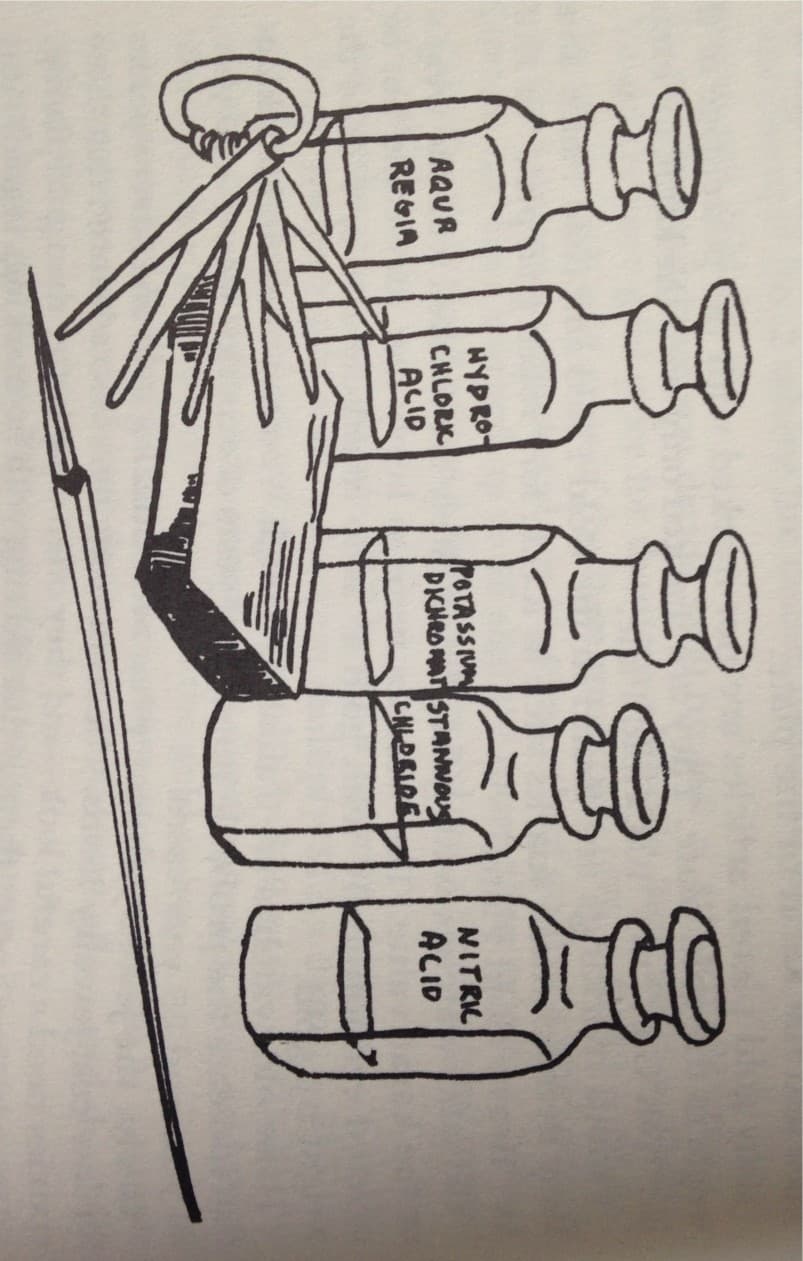
To achieve maximum safety in any metal testing, you will need two acid testing bottles with dip sticks, two flat pieces of unglazed porcelain (one will be for 10K and 14K, and the other will be for 18K), pure-grade nitric acid, a pumice stone, a sample each of 10K, 14k and 18K gold, and a small metal file. (Fig. 5-1).
Fig. 5-1 Testing gold for karatage content requires only a few tools. You'll need two bottles to hold the nitric acid and the Aqua Regia, two pieces of unglazed porcelain, a pumice stone, a small metal file and gold samples consisting or 10K, 14K, 18K, and 24K. All of these items are available at a hardware store or chemical supply house.
The porcelain testing stones can be obtained by using an emery cloth to remove the glaze from a porcelain dish or saucer. A pumice stone can be obtained at a drug store, and hardware stores - or a chemical supply house - generally can supply some nitric acid.
The gold pieces of various karatage are used as comparison pieces to check against unknown metals.
To test for yellow gold, rub the metal in question on the 14K gold testing stone so that it leaves a mark or streak on the touchstone. Then using the acid bottle dip stick, touch some nitric acid on the streak. Base metal (non gold) will appear green or gray, but 14K gold or better will remain unaffected.
Before proceeding to the next karatage test step, take the file and select an inconspicuous spot that will not mar the item's appearance and file a tiny slot through the surface and into the base metal itself. Apply a light touch of nitric acid to the filled slot and watch carefully.
If you see no change at all, the item is 14k gold or better. If a green color appears in the notch it's a base metal or brass: if the color is a silver alloy the notch will show a dark gray color. If the base metal is white and doesn't react with the acid it could be iron or steel.
For further, higher karatage testing the professional approach is to use a chemical called aqua regia. This can be prepared or purchased separately. You can make it yourself with the second bottle of nitric acid by adding a pinch of salt. This converts the acid to aqua regia. Aqua regia is used only on the 18 karat stone.
On the stone with the salt acid, scratch or streak marks made by 10K gold will disappear, a 14K mark will turn dark, and an 18K mark, will remain unaffected.
You can clean the testing stones easily by rubbing them with pumice under running water. The stones can be wiped dry with any tissue.
Just be careful not to use the same area of pumice or the same tissue on both stones. If you wipe off the 18K stone and then use the same tissue on the 10K - 14K stone the latter stone will be useless for any further testing.
That essentially comprises the technique announced by Landis. It is useful not only with yellow gold. It can be used for testing white gold, using white gold items as comparisons for applying streaks. Most white gold jewelry items are stamped with karatage.
Dr. Gerald Wykoff GG CSM
Dr. Gerald Wykoff is GG (Graduate Gemologist), a CSM (Certified Supreme Master gemcutter), educator, and author of several gemology books. He founded the American Society of Gemcutters in the 1980s and served for more than 10 years as the editor of its monthly magazine, American Gemcutter.
Related Articles
How to Calculate the Jeweler’s Markup for Gold Jewelry
Soldering Precious Metals
Relationship Between the Price of Gold and the Price of Your Jewelry
Jewelry Metals 101: Gold, Silver, and Platinum
Never Stop Learning
When you join the IGS community, you get trusted diamond & gemstone information when you need it.
Get Gemology Insights
Get started with the International Gem Society’s free guide to gemstone identification. Join our weekly newsletter & get a free copy of the Gem ID Checklist!